BUSINESS NEWS
J&J’s new ketamine-like depression drug Spravato given to patients
[ad_1]
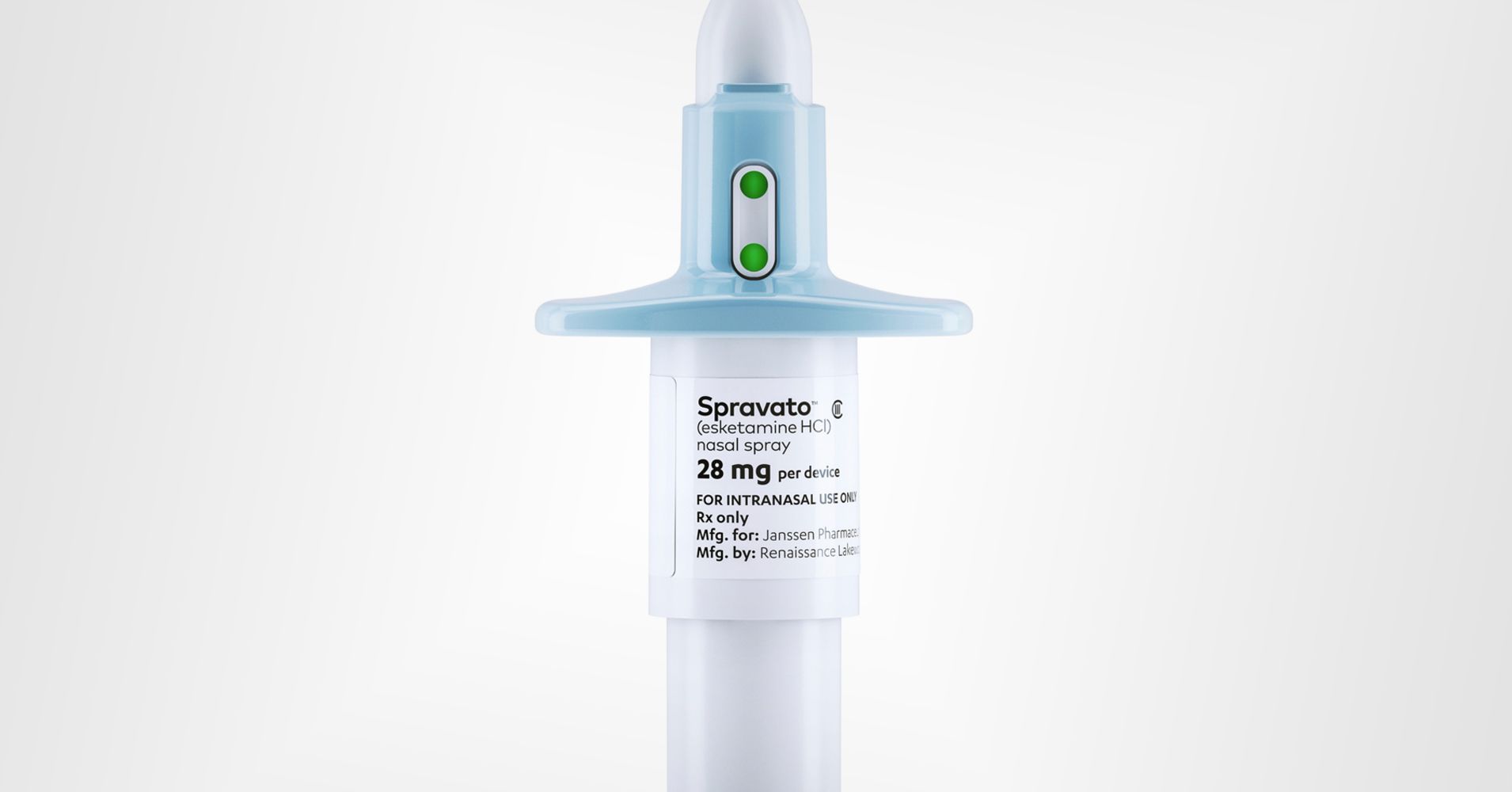
Up to 800 health centers have been approved to administer Johnson & Johnson’s new ketamine-like depression drug, which was cleared for sale last month, and patients are already using it, the company said Tuesday.
Spravato, or esketamine, won federal approval March 5 for treatment-resistant depression. It’s similar to ketamine, a sedative known as the club drug “Special K” that’s increasingly being studied and used to treat depression.
For more on investing in health care innovation, click here to join CNBC at our Healthy Returns Summit in New York City on May 21.
Spravato’s side effects include sedation and dissociation. It also carries the potential risk of misuse and abuse. Acknowledging these factors, the FDA stipulated Spravato must be administered in a medically supervised health-care setting where patients are monitored. Pharmacies, doctor’s offices and clinics also need to be certified.
In a little more than a month, J&J has certified up to 800 sites, putting the company “well on track” with its plans for the year, Jennifer Taubert, executive vice president of pharmaceuticals told analysts on a call Tuesday discussing first-quarter earnings results. She said a number of patients have received their first dose with some receiving multiple doses.
“So we believe that we’re off to a very, very strong start with Spravato, and that it is going to be an important growth driver for us,” she said.
[ad_2]
Source link